VOIS for patients
APrevent® VOIS
VOCAL IMPLANT SYSTEM
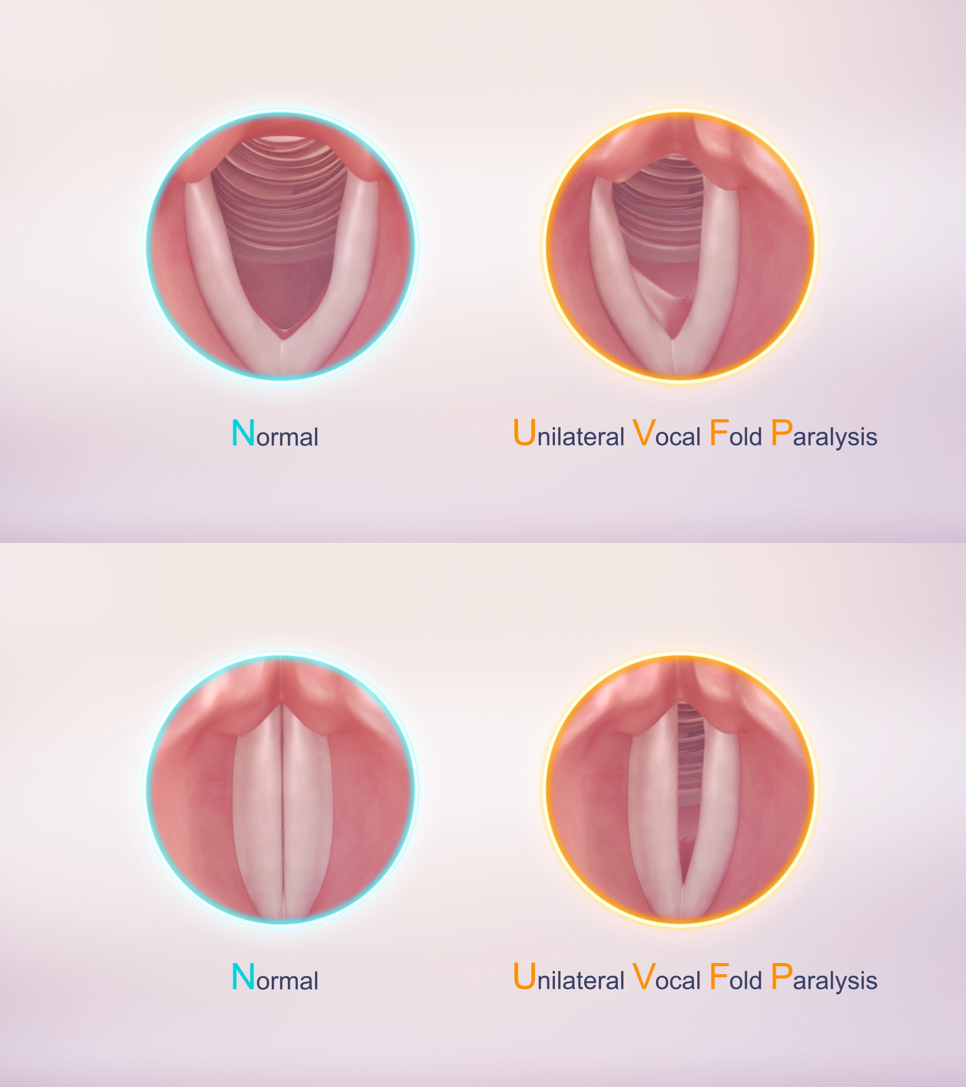
Unilateral Vocal fold paralysis
Unilateral Vocal fold paralysis (UVFP) is the most common neuromuscular disorder in the larynx. It is a condition caused by impairment of the recurrent laryngeal nerve or high vagal lesions, resulting in immobility of one or both vocal folds.
Cause:
- Stroke
- Neurological disorder induced by head and neck, chest or skull base surgery
- Parkinson's disease
- Aging (Presbylaryngis)
Symptoms:
- Dysphonia
- Dysphagia
- Aspiration
Sequels:
- Aspiration Pneumonia
How to treat UVFP?
Medialization Thyroplasty (MT) is the gold standard treatment for glottis insufficiency, by inserting alloplastic or autologous materials into the paraglottic space to achieve glottis closure. In patients with a large glottis gap or high vagal lesions, Arytenoid Adduction (AA) can be performed to reduce the posterior glottis gap and correct the vertical height difference between the vocal folds.
Currently, several products are available in the market, including silastic implants, Gore-Tex stripes, or titanium plates.
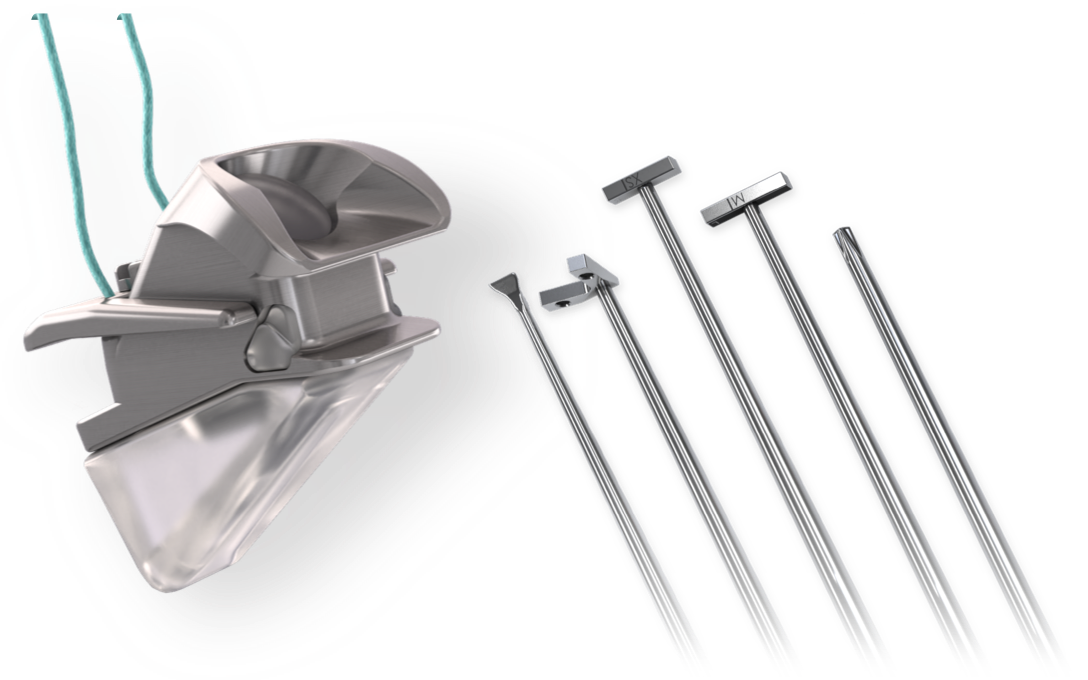
Unlike existing products, APrevent® VOIS provides an innovative technology and solution, it can also be adjusted intra- and postoperatively. APrevent's product is designed to fit individual needs and greatly improves the quality of life.
What's APrevent solution?
“ Advantages of APrevent VOIS ”
- Shorter recovery time
- Minimal foreign body sensation
- Optimized anterior and posterior glottal closure
- Sugery can be done under general or local anesthesia
- Post-operative adjustment at office-based setting without revision surgery
- Standardized, safe, effective surgical procedure
APrevent VOIS is made of silicone and titanium, which are inert and hypoallergenic materials. It is characterized by its unique design for medialization thyroplasty. The goal is to get optimized results with reduced complications and revision rates.
It is the first implant in the market that can be adjusted both intra- and postoperatively. Follow up procedures can be easily performed. The particular shape and unique design of the implant enables a precise adjustment of VOIS, improving voice quality, swallowing and preventing complications from glottic insufficiency.
The implant materials are biocompatible medical grade silicone and titanium and can be implanted through standardized procedure, the latteralleviating pain and shortening of recovery time. Postoperative implant adjustments make it possible to optimize individual needs without revision surgery.
Summary of Safety and Clinical Performance"
“ WHAT PATIENT SAY”

